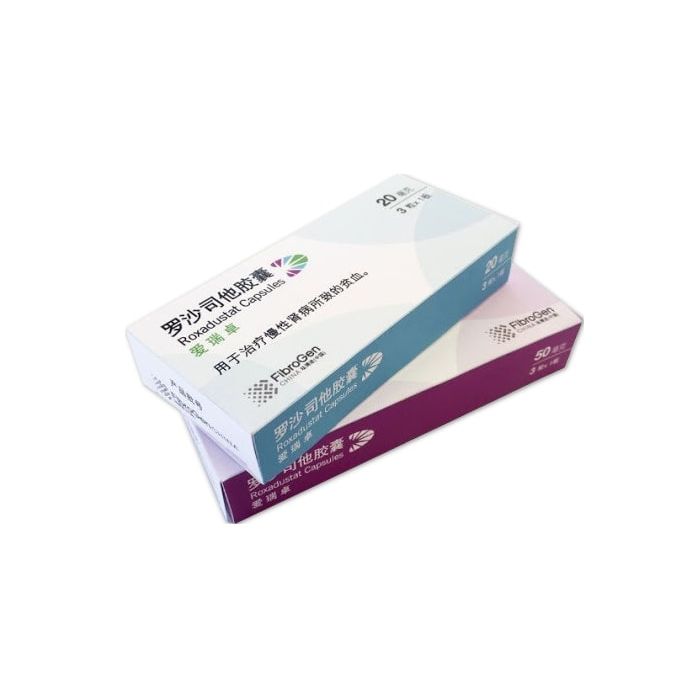
Evrenzo (roxadustat) for sale – Buy Evrenzo (roxadustat) online
What is Evrenzo (roxadustat) for?
Evrenzo (roxadustat) is a medication used for the treatment of anemia associated with chronic kidney disease (CKD) in dialysis patients.
It is available in tablet form containing 20 mg, 50 mg, or 100 mg of roxadustat.[1]
How does Evrenzo (roxadustat) work?
Roxadustat is a hypoxia-inducible factor (HIF) prolyl-hydroxylase (PH) inhibitor. HIF-PH is an enzyme, a type of protein, that is involved in the degradation of HIF. HIF is a protein called a transcription factor that allows for the transcription of genes. This means that they copy a specific DNA sequence into RNA, so that a functional protein can be made.[2]
HIF promotes the transcription of proteins that are involved in the formation of new blood vessels and new red blood cells and hemoglobin. It is activated when there is too little oxygen present in the blood. It makes sure that cells and organs can receive more oxygen.[2,3]
In patients with renal failure, oxygen delivery to the kidneys is too low, as well as oxygen consumption. By blocking HIF-PH, roxadustat promotes the delivery of oxygen, as well as the production of oxygen-carrying red blood cells and hemoglobin.[3]
Where has Evrenzo (roxadustat) been approved?
Evrenzo (roxadustat) was approved by:
- The Pharmaceuticals and Medical Devices Agency (PMDA), Japan on September 20, 2019 for the treatment of anemia in CKD patients dependent on dialysis.[3]
- National Medical Products Administration (NMPA), China on August 22, 2019 in both dialysis-dependent and non-dialysis dependent CKD patients.[4]
- European Medicines Agency (EMA), Europe on August 20, 2021 for anemia associated with CKD.[5]
Please note that this medicine may have also been approved in other regions than the ones we’ve listed. If you have a question about its approval in a specific country feel free to contact our support team.
How is Evrenzo (roxadustat) taken?
The standard dosage is:[1]
- For patients naïve to erythropoiesis-stimulating agents: starting dose of 50 mg of Evrenzo (roxadustat) tablets, taken 3 times per week.
- For patients switching from an erythropoiesis-stimulating agent: starting dose of 70 mg or 100 mg of Evrenzo (roxadustat) tablets, taken 3 times per week.
- For both patient subgroups: second and subsequent doses should be adjusted in line with the patient’s condition.
Please note that the maximum dose does not exceed 3.0 mg/kg body weight.[1]
Evrenzo should be stopped if Hb levels do not increase within 24 weeks.[1]
Complete information about Evrenzo (roxadustat) dosage and administration can be found in the official prescribing information listed in our references section.[1]
Note: Please consult with your treating doctor for personalised dosing and potential drug interactions.
Are there any known adverse reactions or side effects of Evrenzo (roxadustat)?
Common adverse reactions
The most common adverse reactions (≥20% of patients) listed in the prescribing information include:[1]
- Nasopharyngitis
- Vomiting
- Hyperkalemia
Serious adverse reactions
The serious adverse reactions listed in the prescribing information include:[1]
- Shunt occlusion
- Angina pectoris
- Pneumonia
- Myocardial ischaemia
Use in a specific population
Evrenzo (roxadustat) can be fatal for a fetus, it is advised to avoid pregnancies and breastfeeding.[1]
For a comprehensive list of side effects and adverse reactions please refer to the official prescribing information.
References
1. Full prescribing information [PMDA]: Evrenzo (roxadustat) [PDF]
Astellas Pharma Inc., Sept 2019
2. Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway and its Potential for Therapeutic Intervention in Malignancy and Ischemia
Ziello JE, Yale J Biol Med, Jun 2007
3. Evrenzo® (roxadustat) Tablets Approved in Japan for the Treatment of Anemia Associated with Chronic Kidney Disease in Dialysis Patients
Astellas Press Release, Sept 20, 2019
4. AstraZeneca and FibroGen’s roxadustat, not yet filed in U.S., nabs 2nd anemia nod in China
Fierce Pharma press release, Aug 22, 2019
5. Evrenzo product information
EMA, Aug 2021



Reviews
There are no reviews yet.